What Is Relative Atomic Mass
The atomic mass is carried by the atomic nucleus which occupies only about 10 -12 of the total volume of the atom or less but it contains all the positive charge and at least 9995 of the total. The atomic mass is carried by the atomic nucleus which occupies only about 10 -12 of the total volume of the atom or less but it contains all the positive charge and at least 9995 of the total.

Definition Of Isotopes And Relative Atomic Mass Solutions
La relazione tra massa quantità di sostanza volume e numero di particelle.
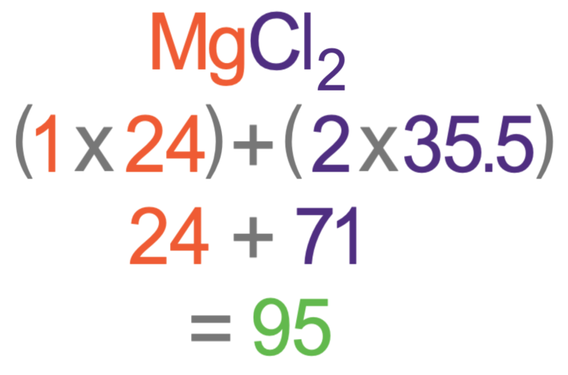
. The atomic weights are available for elements 1 through 118 and isotopic compositions or abundances are given when appropriate. The relative atomic masses of the isotopes data were published by M. The relative atomic mass.
Relative Atomic Mass - Key takeaways. The atomic mass of carbon is 12011. M u is defined as being 1 12 of the mass of a.
Examples of Atomic Mass. What is Atomic Mass. The related quantity relative molecular mass as defined by IUPAC is the ratio of the mass of a molecule to the unified atomic mass unit also.
It is the ratio of the average mass per atom of an element from a given sample to 112 the mass of a carbon-12 atom. The most common isotope of hydrogen is protium an atom that consists of a proton or a proton and an electron. The relative atomic mass of carbon is 12 while the relative atomic mass of magnesium is 24.
The relative atomic mass A r of an element is the average mass of the naturally occurring atoms of the element. Visit BYJUS to learn more about it. Water is 11 hydrogen by mass but 67 hydrogen by atomic percent and these numbers along with the complementary numbers for oxygen in water are the largest contributors to overall mass and atomic.
- The relative atomic mass of an element is the average mass of the naturally-occurring isotopes of the element relative to the mass of an atom of 12C. Pfeiffer in The AME2012 Atomic Mass Evaluation. The average atomic mass sometimes called atomic weight of an element is the weighted average mass of the atoms in a naturally occurring sample of the element.
Atomic mass is the sum of all the protons neutrons and electrons in a single atom or molecule. Relative atomic mass symbol. A r is a measure of how heavy atoms are.
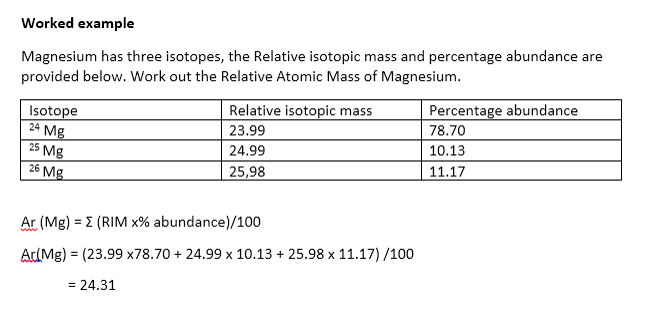
The formula for relative atomic mass is isotope mass x isotope abundance 100. The element rhenium consists of two isotopes 185 Re and 187 Re in the atomic ratio of 23. It might be given in Atomic Ratio or Abundance.
An atomic mass unit symbolized AMU or amu is defined as precisely 112 the mass of an atom of carbon-12. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element. This quantity takes into account the percentage abundance of all the isotopes of an element which exist.
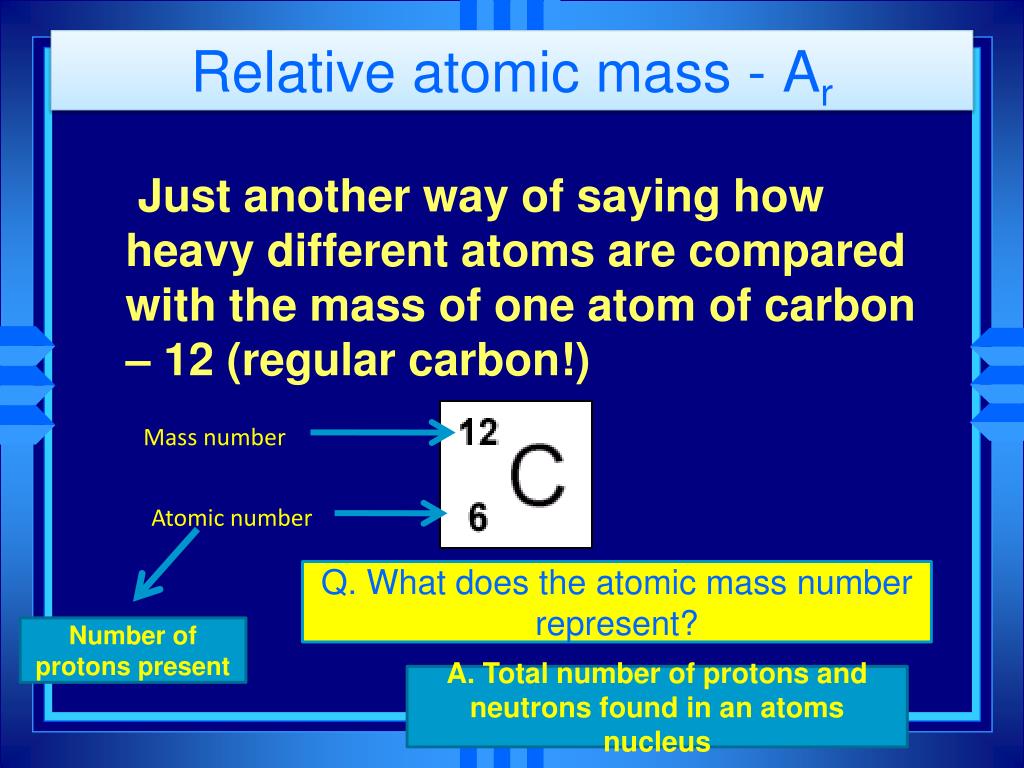
Mass number is the sum of protons and neutrons present in the nucleus of an atom and it is a whole number value while relative atomic mass is the ratio of the mass of an element to the 112 th of the mass of a carbon atom. Atomic mass unit AMU or amu. The relative atomic mass of an element symbol A r is the relative mass of its atoms compared to the mass of a carbon-12 atom.
The relative atomic mass A r of an element is calculated from. La massa atomica m a di un atomo è la massa di quel singolo atomo espressa in unità di massa. It is measured in daltons Da or u.
Join an activity with your class and find or create your own quizzes and flashcards. The atomic mass of hydrogen is 10079. Tuttavia poiché può assumere valori compresi tra i 10 25 kg e i 10 27 kg è solitamente espressa in Da dalton derivata dal nome di John Dalton o unità di massa.
If the substance is made of simple molecules this mass may also be called the relative molecular mass. For example the atomic mass of iron is 55845 u. In other words a relative atomic mass tells you the number of times an average atom of an element from a given sample is heavier than one-twelfth of an.
A relative atomic mass also called atomic weight. The molecular mass m is the mass of a given molecule. The A r values for elements are given in the periodic table.
The relative atomic mass of an element shows its mass compared with the mass of atoms of other elements. However the mass of an electron is so small it is considered negligible and not included in the calculation. The relative amounts of each element vary by individual mainly due to differences in the proportion of fat muscle and bone in their body.
The symbol for relative formula mass is M r. Average masses are generally expressed in unified atomic mass units u where 1 u is equal to exactly one-twelfth the mass of a neutral atom of carbon-12. The formula for relative atomic mass is.
Though technically incorrect the term is also often used to refer to the average atomic mass of all of the isotopes of one element. Most carbon atoms consist of six protons and six neutrons. The atomic mass or relative isotopic mass refers to the mass of a single particle and therefore is tied to a certain specific isotope of an element.
Calculate the relative atomic mass of rhenium to three significant figures. The carbon-12 C-12 atom has six protons and six neutrons in its nucleus. Different molecules of the same compound may have different molecular masses because they contain different isotopes of an element.
The mass number is a count of the total number of protons and neutrons in an atoms nucleus. How To Calculate Relative Atomic Mass Chemical Calculations Chemistry FuseSchoolDo you want to know how to calculate relative atomic mass. Atomic mass is the weighted average mass of an atom of an element based on the relative natural abundance of that elements isotopes.
Hydrogen atomic number 1 is the element that has the lowest atomic mass. Relative mass is the mass of an atom or molecule compared to that of 112 of a carbon-12 atom. The relative formula mass of a substance is the sum of the relative atomic masses of the elements present in a formula unit.
Of an element is the average mass of its atoms compared to 112th the mass of a carbon-12 atom. What is relative formula mass and relative molecular mass. Relative atomic mass Ar is the weighted average of the masses of the isotopes of an element compared to 112 of the mass of the carbon-12 atom.
This second definition is. RAM can be in decimal form. Lets check out an exam-based question that gives you the information in terms on Atomic Ratio.
Sometimes abbreviated RAM or ram also known by the deprecated synonym atomic weight is a dimensionless physical quantity defined as the ratio of the average mass of atoms of a chemical element in a given sample to the atomic mass constantThe atomic mass constant symbol.

What Is Relative Atomic Mass Chemical Formula And Equation Youtube

Calculating Relative Atomic Mass Support Sheet Teaching Resources

Jkgx1ntqqz0skm

Relative Atomic Mass Molecular Mass O Level Chemistry Notes
If The Relative Atomic Mass Of Oxygen Is 64 Units The Molecular Mass Of Co Becomes

Relative Atomic Mass Atomic Mass Unit Examples Relative Atomic Mass Relativeatomicmass Atomicmassunit Chemistry Najamacademy By Najam Academy Facebook
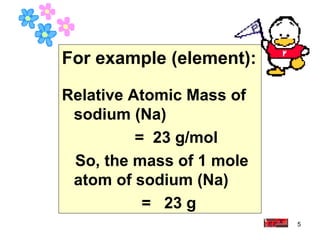
3 3 B Relative Atomic Mass
How To Calculate Relative Atomic Mass Quora
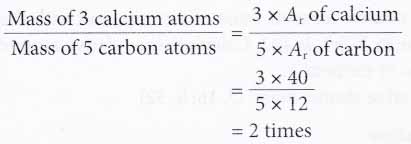
What Is The Relative Atomic Mass And Relative Molecular Mass Of An Element A Plus Topper

Relative Atomic Mass A Question Science By Degrees

Relative Atomic Mass And Atomic Mass Unit Youtube

Relative Atomic Masses And Half Lives Of Selected Radionuclides Download Table
Made Easy Chemistry How To Calculate Relative Atomic Mass
Atomic Mass Vce Chemistry
Ppt Relative Atomic Mass A R Powerpoint Presentation Free Download Id 513605

Relative Atomic Mass Shalom Education

Pin On Teaching Ideas